Make sure you have your authentication code ready in advance of Whenever your confirmation statement is thanks. It is because, if you need it to get resent to you, it can take as much as five Doing work days to reach by article, and in many cases for a longer time during fast paced durations. The authentication code cannot be provided by email or p
FBD principle Secrets
Besides enhanced efficiency, these dryers can cope with a wide range of particle measurements, styles, and densities, generating them quite functional. The rigorous mixing and huge surface area exposed to the heat also lead to comparatively shorter drying times, giving elevated throughput.We goal to reveal the guarded secrets of the engineering fie
process validation Fundamentals Explained
Process validation is actually a vital Component of high quality assurance while in the manufacturing sector. It includes the gathering and Assessment of data in order that a process consistently provides products which fulfill predetermined specifications and excellent specifications.Process validation is usually outlined as the documented evidenc
About Filling and Sealing Operation in Sterile Manufacturing
Is the applying truly a sterile application and how do they come across the proper CDMO to manufacture their solution safely and effectively? Down below We're going to overview the […]Secondly, these equipment Engage in an important position in ensuring uniformity in Every bottle’s filling, that is crucial for pharmaceutical products and soluti
A Simple Key For Filling in Sterile Manufacturing Unveiled
For example, in 2020, the FDA declared that some prescription drugs have been contaminated having a ‘likely human carcinogen called NMDA.’ This transpired due to the fact there was a lack of controls to keep up an aseptic atmosphere. Patented technological know-how results in two welds to circumvent environmental contamination from discarded t
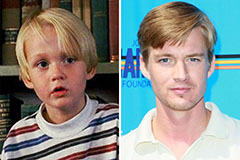 Mason Gamble Then & Now!
Mason Gamble Then & Now! Alexa Vega Then & Now!
Alexa Vega Then & Now! Michael Jordan Then & Now!
Michael Jordan Then & Now! Catherine Bach Then & Now!
Catherine Bach Then & Now! The Olsen Twins Then & Now!
The Olsen Twins Then & Now!